Research
Immunometabolism
We are interested how metabolic signals and the cellular metabolism impacts macrophage function in vivo and in the immunometabolic crosstalk of macrophages with tissues.
Driven by recent technological breakthroughs, “immunometabolism” has become an exciting area of biomedical research and is a focus of our lab. We are investigating metabolic crosstalks between macrophages and tissue cells to shape tissue homeostasis and maintain tissue integrity. We hypothesize that tissue integrity is maintained by macrophages sensing tissue status through metabolites, and metabolites themselves serve as signals in cell-cell communication. In this regard, we are evaluating roles for macrophages to provide nutritive support in the form of metabolites such as polyamines to epithelial tissue cells and how this influences chronic inflammatory diseases and cancer. We suggest that macrophages function as "metabolic factories" that support the metabolism of other cells. We are investigating novel metabolites that are secreted by macrophages and have nutritive or immunomodulatory functions.

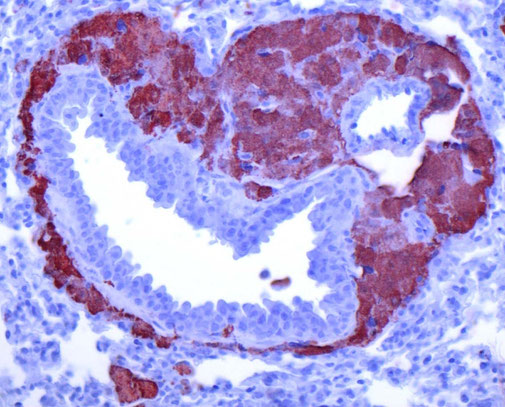
Sarcoidosis and other Granulomatous diseases
We are working on basic cellular processes that drive granuloma formation in macrophages. Granulomatous diseases such as sarcoidosis or tuberculosis are a important health problem with still insufficient treatment options. Granulomas are formed by macrophages that transform into epithelioid cells that increase in size and aggregate. We could show that chronic activation of mTORC1 in macrophages drives granuloma formation, marking disease progression in sarcoidosis. This finding led to a novel therapeutic approach using mTOR inhibitors, such as sirolimus, to treat sarcoidosis, which has shown promising results in clinical trials, particularly for cutaneous forms of the disease. We are currently investigating how lipid processes and organ crosstalk contributes to granulomatous inflammation and how granulomatous macrophages promote fibrosis.
mTOR in innate immunity
Myeloid phagocytes such as monocytes, macrophages, or dendritic cells are at the basis of controlling immune and homeostatic responses in all tissues of the body.
Our group has shown previously that the mechanistic target of rapamycin (mTOR) pathway is crucially implicated in these innate reactions and serves as a decision maker to control the cellular response to pathogens by regulating the expression of inflammatory mediators such as cytokines, chemokines or interferons.
A focus of our group is to study the molecular mechanisms of how mTOR complex 1 (mTORC1) and mTORC2 influences innate immune activation in macrophages and dendritic cells induced by pathogens or environmental signals and its consequences for the adaptive immune response. We study how inhibition of mTOR, which is an established therapy in basic organ transplantation and cancer, modulates the inflammatory response and how the mTOR pathway incorporates environmental and nutritional signals to regulate host immunity and tissue homeostasis. These processes are studied by biochemical and molecular approaches and in mouse models in vivo by analyzing mice with tissue-specific knockouts for certain molecules of the mTOR pathway. Additionally, we corroborate our data by analyzing human tissues and samples.


Thomas Weichhart Lab at the Medical University of Vienna, Center for Pathobiochemistry & Genetics, Vienna, Austria